
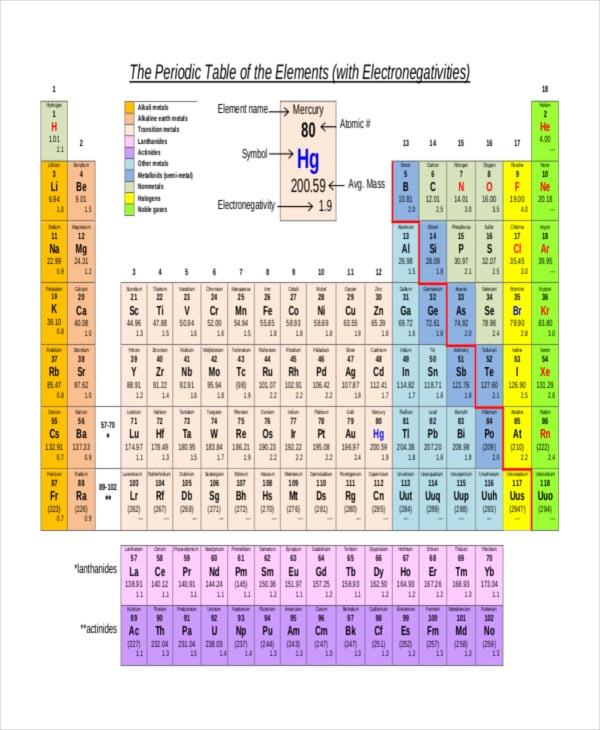
To calculate the electronegativity of a molecule you have to subtract the smaller electronegativity value from the larger one.Looking at the elements in the periodic table, in general, electronegativity of elements increases from:.A dipole is a difference in charge between two bonded atoms that is caused by a shift in electron density in the bond.
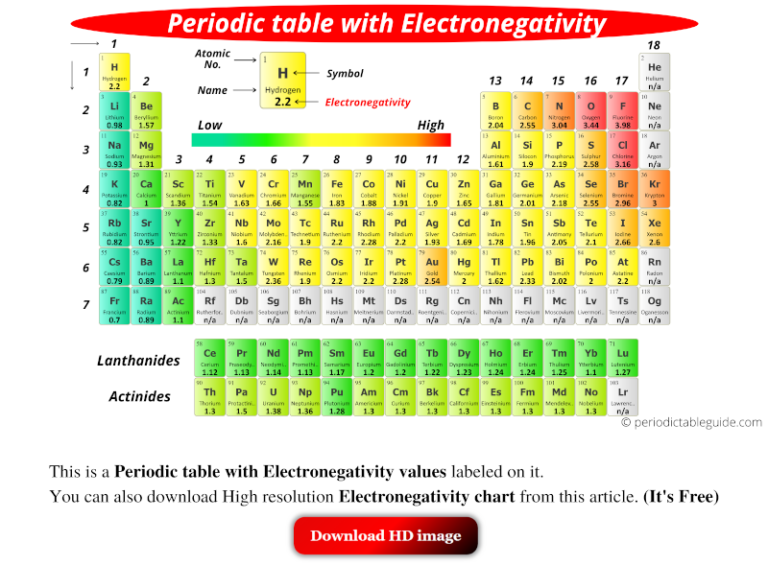
A bond is non-polar if the two atoms have similar electronegativities.The more electronegative atom pulls the bonding pair of electrons towards itself.
 The larger the difference in electronegativity of the elements forming the bond, the higher the chance that the bond will be ionic. Diatomic molecules have a difference of zero in electronegativity, as both atoms have the same value of electronegativity. The Pauling scale can be used to predict the percentage ionic or covalent character of a chemical bond. As you go across a period in the periodic table, the electronegativity increases. As you go down a group in the periodic table, the electronegativity decreases. The factors that affect electronegativity are atomic radius, nuclear charge, and shielding. If there is an electronegativity difference between 0.5 and 1.9, then the bond will be a polar covalent bond. If the difference is less than 0.5 then the bond will be a non-polar covalent bond. Usually, if the difference in electronegativity is greater than 2.0, the bond is likely to be ionic. Compounds such as magnesium oxide (MgO), sodium chloride (NaCl), and calcium fluoride (CaF 2) are examples of this. The atom which loses its electron(s) becomes a cation which is a positively charged species, whilst the atom which gains the electron(s) becomes an anion, which is a negatively charged species. This occurs when there is a large enough difference between electronegativity values of the two atoms in a molecule the least electronegative atom transfers its electron(s) to the more electronegative atom. Due to this, partial charges appear on the molecule, since the more electronegative atom gains a partial negative charge, while the less electronegative atom gains a partial positive charge.Īn ionic bond is formed when one atom completely transfers its electrons to another atom which gains the electrons. In this case, the more electronegative atom (the atom with the higher value in the Pauling scale) attracts the bonding pair of electrons towards itself. This results in the formation of a polar covalent bond. However, when atoms with different electronegativities form a molecule, the sharing of electron density is not equally distributed between the atoms. This results in a stronger attraction for the bonding pair of electrons. As a result of this, the atomic radius decreases because the outermost shell is pulled closer to the nucleus, so the distance between the nucleus and the outermost electrons decreases. However, shielding remains constant since no new shells are being added to the atoms, and electrons are being added to the same shell each time. The nuclear charge increases because the number of protons in the nucleus increases. Electronegativity across a periodĪs you go across a period in the periodic table, electronegativity increases. This leads to an increase in the distance between the nucleus and the outermost electrons, meaning that there is a weaker force of attraction between them.
The larger the difference in electronegativity of the elements forming the bond, the higher the chance that the bond will be ionic. Diatomic molecules have a difference of zero in electronegativity, as both atoms have the same value of electronegativity. The Pauling scale can be used to predict the percentage ionic or covalent character of a chemical bond. As you go across a period in the periodic table, the electronegativity increases. As you go down a group in the periodic table, the electronegativity decreases. The factors that affect electronegativity are atomic radius, nuclear charge, and shielding. If there is an electronegativity difference between 0.5 and 1.9, then the bond will be a polar covalent bond. If the difference is less than 0.5 then the bond will be a non-polar covalent bond. Usually, if the difference in electronegativity is greater than 2.0, the bond is likely to be ionic. Compounds such as magnesium oxide (MgO), sodium chloride (NaCl), and calcium fluoride (CaF 2) are examples of this. The atom which loses its electron(s) becomes a cation which is a positively charged species, whilst the atom which gains the electron(s) becomes an anion, which is a negatively charged species. This occurs when there is a large enough difference between electronegativity values of the two atoms in a molecule the least electronegative atom transfers its electron(s) to the more electronegative atom. Due to this, partial charges appear on the molecule, since the more electronegative atom gains a partial negative charge, while the less electronegative atom gains a partial positive charge.Īn ionic bond is formed when one atom completely transfers its electrons to another atom which gains the electrons. In this case, the more electronegative atom (the atom with the higher value in the Pauling scale) attracts the bonding pair of electrons towards itself. This results in the formation of a polar covalent bond. However, when atoms with different electronegativities form a molecule, the sharing of electron density is not equally distributed between the atoms. This results in a stronger attraction for the bonding pair of electrons. As a result of this, the atomic radius decreases because the outermost shell is pulled closer to the nucleus, so the distance between the nucleus and the outermost electrons decreases. However, shielding remains constant since no new shells are being added to the atoms, and electrons are being added to the same shell each time. The nuclear charge increases because the number of protons in the nucleus increases. Electronegativity across a periodĪs you go across a period in the periodic table, electronegativity increases. This leads to an increase in the distance between the nucleus and the outermost electrons, meaning that there is a weaker force of attraction between them. 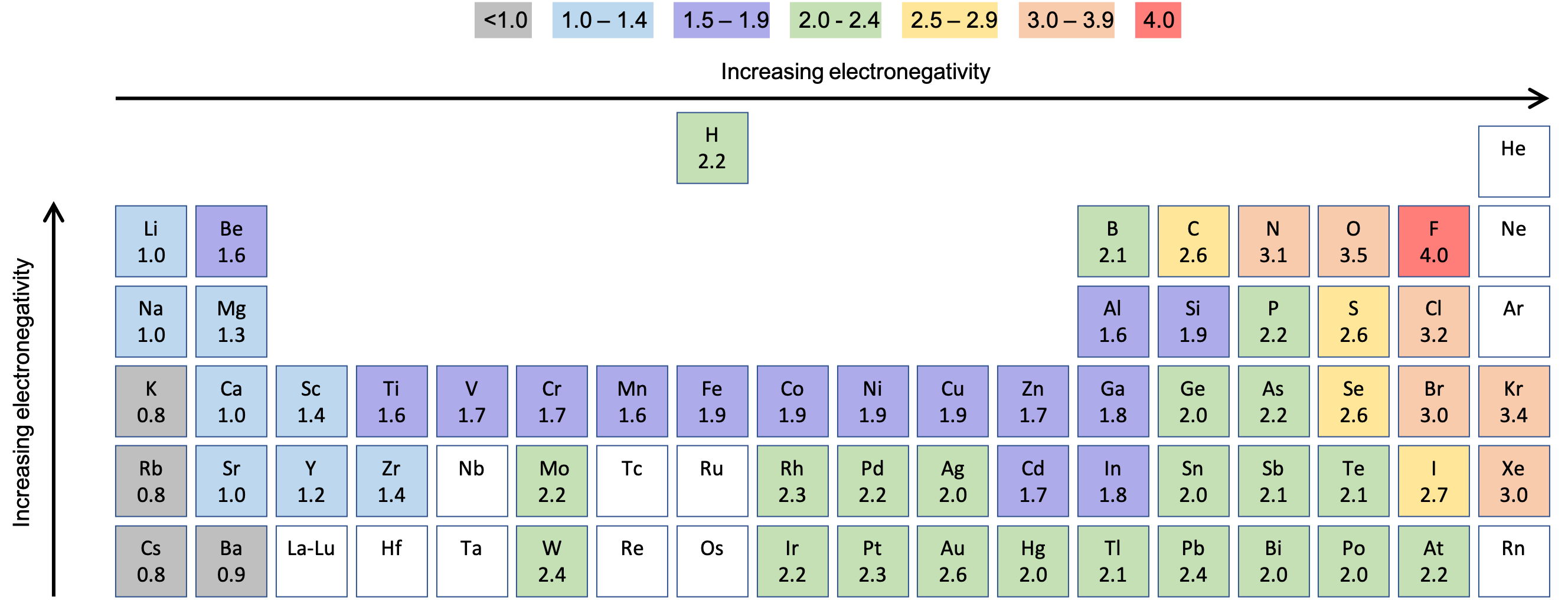
The atomic radius of the atom increases as you go down the group since you are adding more shells of electrons, which makes the atom larger. However, the effect of shielding is also increased as there is an extra filled electron shell in each element going down a group. The nuclear charge increases as protons are added to the nucleus. Electronegativity down a groupĮlectronegativity decreases g oing down a group in the periodic table. Let's look at some basic trends in electronegativity, which generally hold true in the periodic table. Electronegativity trends in the periodic table Therefore, the addition of extra subshells and inner shells causes outer electrons to be less attracted to the nucleus. This is because the electrons of the inner shells 'shield' the outer electrons from the attraction of the nucleus, causing the attraction between the outer electrons and the nucleus to decrease. If you increase the number of subshells and inner shells of an atom, the electronegativity decreases. Reaction Quotient and Le Chatelier's Principleīeware! Do not confuse nuclear charge with an element or compound having a charge.Elemental Composition of Pure Substances.Application of Le Chatelier's Principle.Intramolecular Force and Potential Energy.Variable Oxidation State of Transition Elements.Transition Metal Ions in Aqueous Solution.








 0 kommentar(er)
0 kommentar(er)
